Medical Device SaaS
In the fast-paced medical device industry, companies are often hampered by the intricate maze of regulations and quality control standards necessary to bring their products to market. Recognizing this challenge, we set out to develop a sophisticated SaaS solution aimed at streamlining the design controls and Quality Management Systems (QMS) specifically tailored for medical device workflows.
Problem
The journey for medical device companies from concept to market is fraught with regulatory complexities and stringent quality control requirements that can be daunting, especially for newcomers. Understanding the specific needs of users and ensuring compliance with FDA (or equivalent) standards involves a steep learning curve. Additionally, the current landscape lacked a unified platform that could seamlessly integrate design controls with quality management systems.
Solution
To tackle these challenges head-on, we developed a comprehensive SaaS platform that integrated linked product design controls with a robust QMS, simplifying the FDA approval process and streamlining post-approval quality management.
Details
Role
Senior Manager, Product
Industry
Medical Tech, SaaS
Tools
Figma, Alteryx, Python
Skills
User Experience Design, Data Analysis, User Research
Actions
Market Analysis, User Interviews, Product Roadmapping, Wireframing
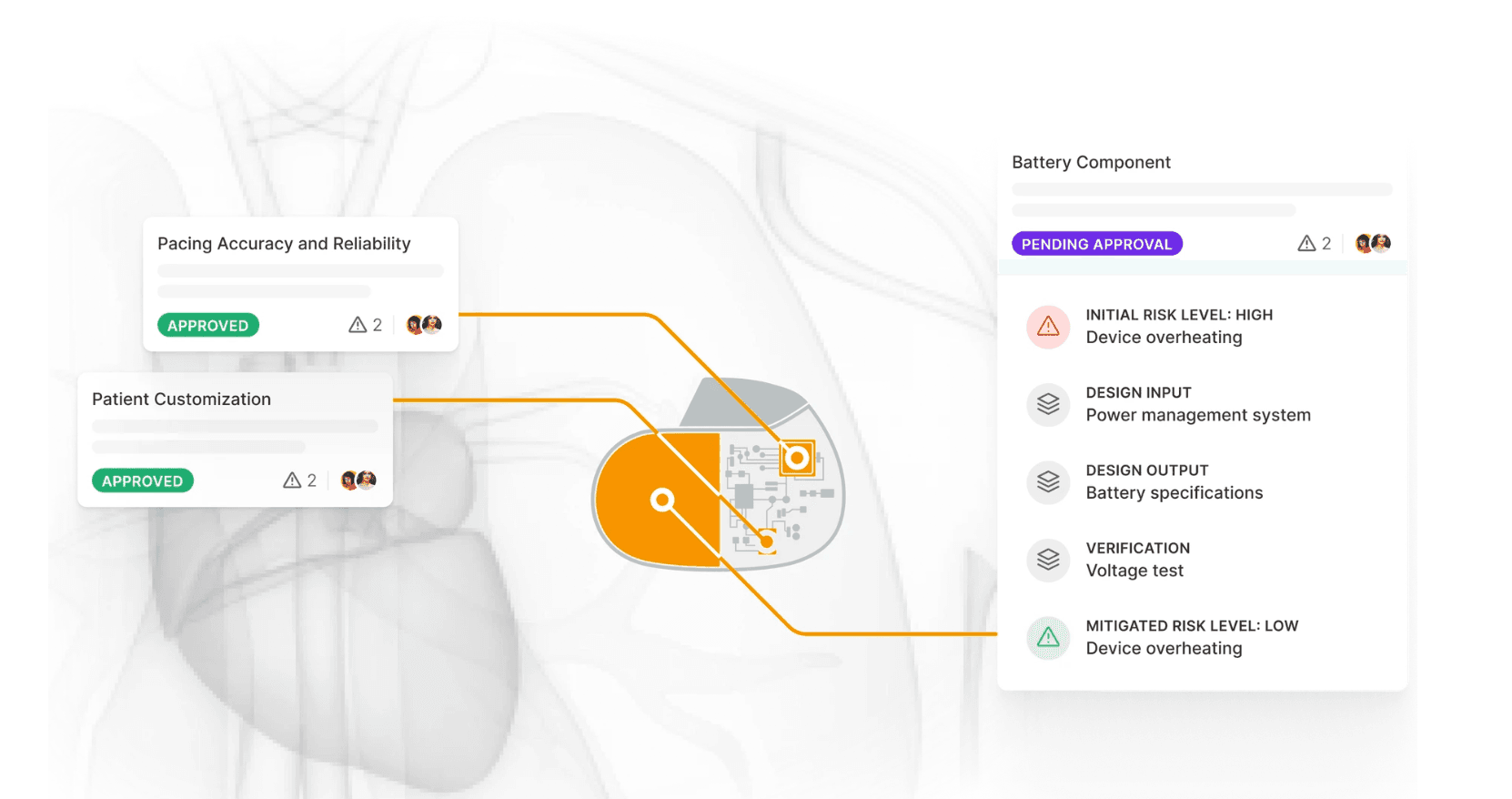
Result
The development was user-centered, informed by dozens of user interviews. The result was a platform that minimized cognitive load, ensured regulatory compliance, and enhanced overall productivity in the medical device manufacturing and quality control process.
